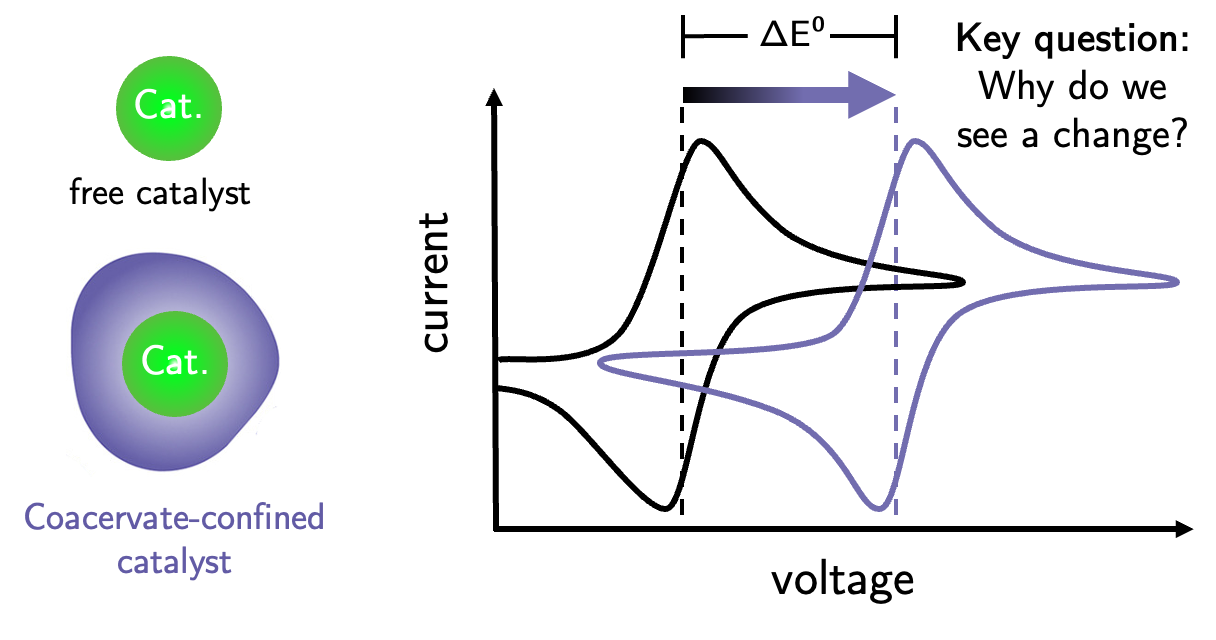
In our lab, we use electrochemistry to investigate how reactant confinement into biological droplets, called coacervates, alters the thermodynamics of a reaction. Liquid-liquid phase separation, or coacervation, is a general phenomenon that can occur in biological systems in response to stimuli such as heightened stress levels or small-molecule substrate concentrations like ATP. The second “coacervate” phase is often formed by the association of poly-cationic and -anionic molecules and exhibits altered properties from the bulk (e.g. a change in pH, small-molecule enrichment or exclusion, depletion of water) in order to facilitate a response. An aspect of particular interest is the enabling or acceleration of chemical transformations by coacervates, thus posing the hypothesis that the first proto-enzymes were coacervate droplets.
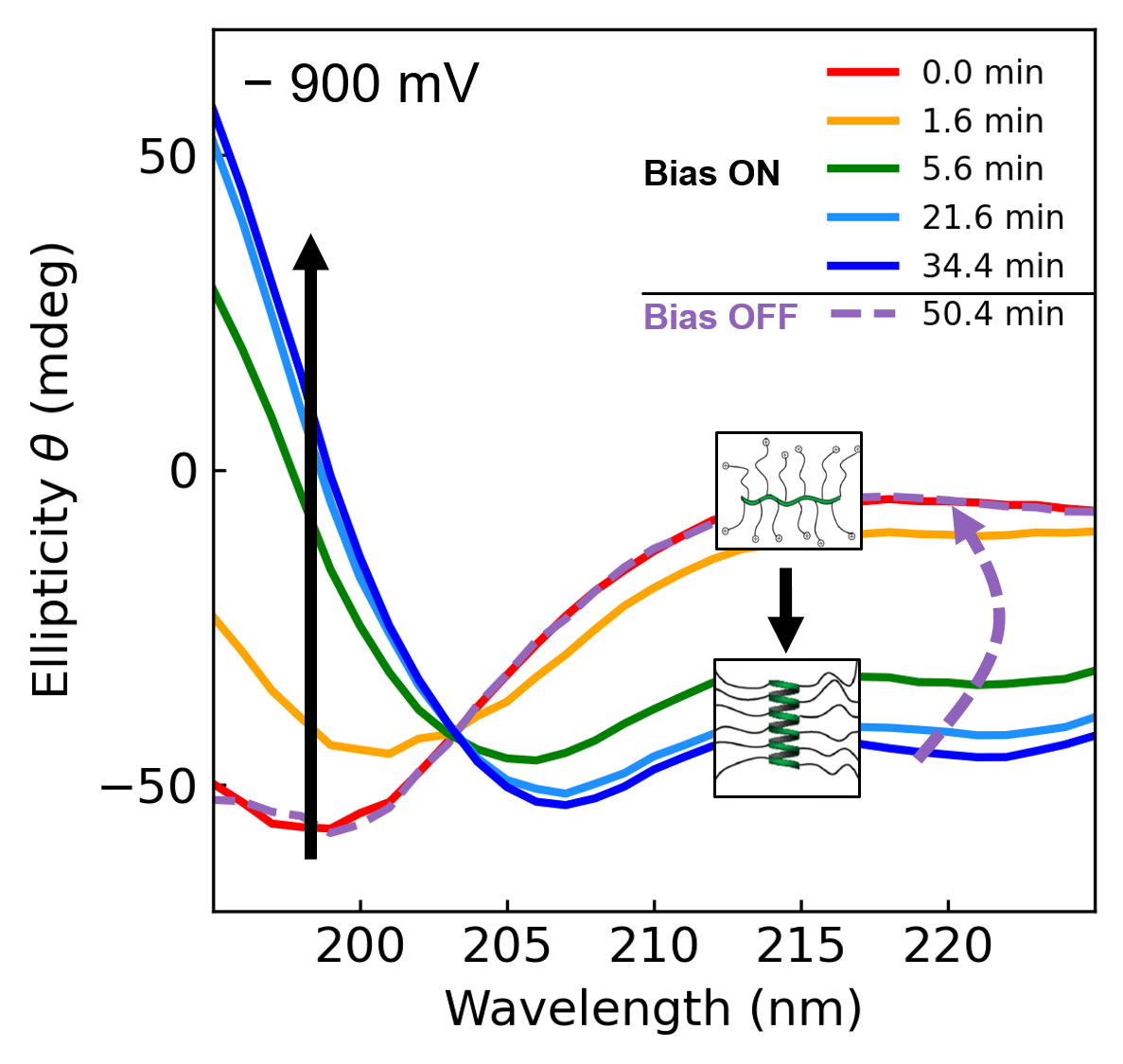
In collaboration with the Morse and Gordon labs, we use electrochemistry as a tool to manipulate the charge state of peptides and proteins to investigate how electrostatic interactions regulate protein structures and functions. In vivo, modulation of charges can occur directly on amino acids by pH or via post-translational modifications such as phosphorylation, often exhibiting a switch-like response of signal transducing systems. By combining electrochemistry with in situ spectroscopy techniques, we are able to finely tune the charge state of proteins and follow their conformational changes and assembly in real-time. This method allows us to study dynamic processes in biomolecular systems and gain fundamental insights into protein machinery.